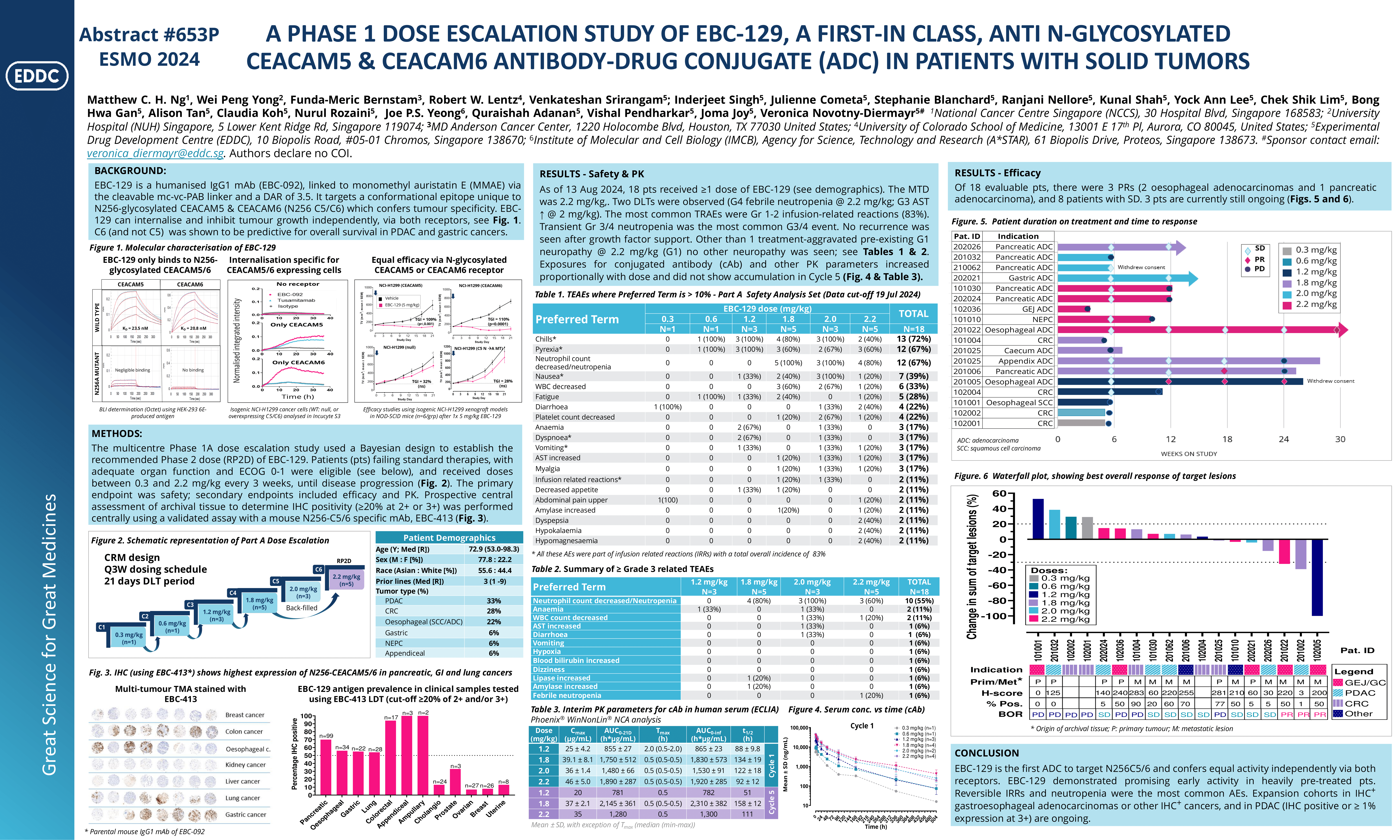
In September 2024, we showcased our phase 1 dose escalation study for EBC-129 (NCT05701527) at European Society for Medical Oncology (ESMO) Congress.
Further to the ESMO poster presentation, we are happy to announce that we have completed enrolment of the pancreatic ductal adenocarcinoma (PDAC) cohort in the ongoing dose expansion part of EBC-129’s Phase 1 study!
Enrollment in cohorts with gastroesophageal adenocarcinomas and the cohort with other IHC positive solid tumours is ongoing.
Find more details about trial at:
Study Details | A Study of EBC-129 in Advanced Solid Tumours | ClinicalTrials.gov